Amikacin Sulphate IP/BP/USP
Amikacin Sulphate IP/BP/USP Specification
- Indication
- Treatment of serious infections caused by Gram-negative bacteria
- Salt Composition
- Amikacin Sulphate IP/BP/USP
- Pacakaging (Quantity Per Box)
- Customised as per order
- Origin of Medicine
- Synthetic
- Dosage Form
- Injection or Infusion after reconstitution
- Drug Type
- Medicine Raw Materials
- Ingredients
- Amikacin Sulphate
- Physical Form
- Powder
- Function
- Antibiotic
- Recommended For
- Bacterial Infections
- Dosage
- As prescribed by physician
- Dosage Guidelines
- As Per Physician Instruction
- Suitable For
- Adults and Children
- Quantity
- Bulk Packing Available
Amikacin Sulphate IP/BP/USP Trade Information
- FOB Port
- All Indian Port
- Payment Terms
- Telegraphic Transfer (T/T), Western Union
- Delivery Time
- 7 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- As Per Standard Packing
- Main Export Market(s)
- Australia, North America, Eastern Europe, Central America, Middle East, South America, Asia, Western Europe, Africa
- Certifications
- COA, GMP
About Amikacin Sulphate IP/BP/USP
This product is a semi-synthetic derivatives of kanamycin sulfate, white or almost white crystalline powder, almost odorless, tasteless. This product is soluble in water, almost insoluble in ethanol. Specific rotation to take this product, accurately weighed, dissolved in water and diluted into 1ml each containing 20mg of the solution, according to the law, the specific rotation of +97 degrees to +105 degrees.
- English name: Amikacin
- Molecular Formula:C22H43N5O13
- Molecular Weight:585.6025
- CAS Registry Number:37517-28-5
- Appearance:white crystalline powder
Packaging details:
| Packaging details: | 25kg/drum with double plastic bags inside; packed in a cardboard drum or fiber HDPE drum. |
| Storage: | Stored in a clean, cool, dry area; keep away from moisture and strong, direct light/heat |
| Shelf Life: | 5 years if sealed and store away from direct sun light. |
Pharmaceutical-Grade Purity and Compliance
Our Amikacin Sulphate is manufactured to achieve 99% purity and meets stringent IP, BP, and USP standards. This ensures reliable quality for pharmaceutical formulation, particularly in injectable antibiotics. Each batch is carefully screened to provide customers with consistent and compliant medicine raw materials.
Diverse Applications in Infection Control
Primarily used in the formulation of injectable antibiotics, Amikacin Sulphate is recommended for combating serious Gram-negative bacterial infections. Its broad utility in hospital settings makes it a preferred ingredient for tailored treatments, effective for adult and pediatric patients under physician guidance.
Custom Packaging & Safe Handling
Available in large bulk packages and with custom packaging as per order, Amikacin Sulphate is prepared and handled using industry best practices. To ensure longevity and effectiveness, it should be stored in a cool environment, away from moisture and contaminants, as per standard pharmaceutical guidelines.
FAQs of Amikacin Sulphate IP/BP/USP:
Q: How is Amikacin Sulphate IP/BP/USP used in pharmaceutical applications?
A: Amikacin Sulphate IP/BP/USP is widely used as a raw material in the formulation of injectable antibiotics. After reconstitution, it is administered as an injection or infusion for treating severe infections caused by Gram-negative bacteria.Q: What are the recommended storage conditions for Amikacin Sulphate powder?
A: To maintain product stability and potency, Amikacin Sulphate should be stored in a cool, dry environment, away from direct sunlight and moisture. Proper storage helps preserve its 36-month shelf life.Q: When should dosage of Amikacin Sulphate be determined?
A: The correct dosage and administration schedule for Amikacin Sulphate are determined by a physician based on patient-specific factors, such as age, medical condition, and infection severity. Never self-administer; always follow healthcare professional instructions.Q: Where is Amikacin Sulphate manufactured and can it be supplied in bulk?
A: Our Amikacin Sulphate is manufactured in India, available for export and bulk supply with customizable packaging quantities to meet client requirements worldwide.Q: What are the primary benefits of using Amikacin Sulphate in medical formulations?
A: Amikacin Sulphate is valued for its high purity, effectiveness against Gram-negative bacteria, and compliance with major pharmacopeial standards, making it a reliable and safe choice for injectable antibiotic preparations.Q: What is the process for preparing Amikacin Sulphate for clinical use?
A: Amikacin Sulphate, as a powder, is reconstituted with a suitable solvent by qualified personnel before being administered as an injection or infusion, following established medical guidelines.Q: Who can be treated with Amikacin Sulphate formulations?
A: Formulations containing Amikacin Sulphate are suitable for both adults and children, but must always be used under medical supervision to ensure safety and efficacy against bacterial infections.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Analgesic Drug & Active Pharmaceutical Ingredient Category
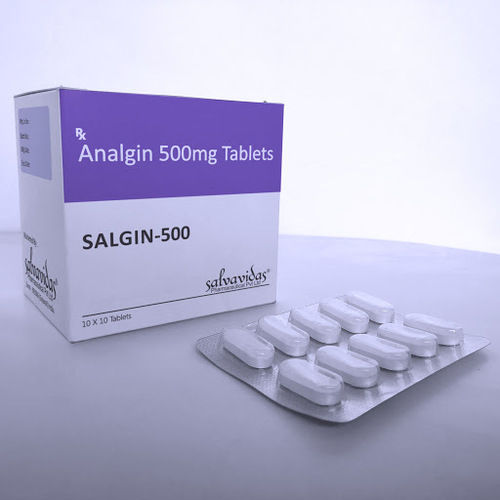
Analgin Tablets
Storage Instructions : Store in Cool
Formulations Form : Tablets
Dosage Guidelines : 12 tablets as needed, do not exceed prescribed dose
Formulations Type : General Drugs
Drug Type : Other, General Drugs
Ingredients : Metamizole Sodium (Analgin)
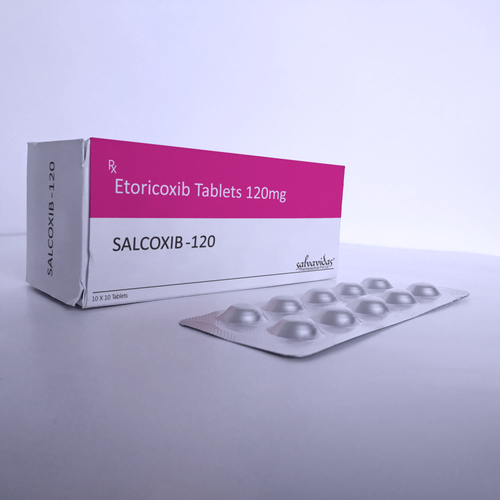
Etoricoxib Tablets
Storage Instructions : Store in Cool
Formulations Form : Tablets
Dosage Guidelines : Take with water, follow physician instructions
Formulations Type : General Drugs
Drug Type : Other, General Drugs
Ingredients : Etoricoxib
Avobenzone Ip/bp/usp
Storage Instructions : Store in Cool
Formulations Form : Powder
Dosage Guidelines : As per formulation or professional guidance
Formulations Type : Medicine Raw Materials
Drug Type : Other, External Use, Pharmaceutical Raw Material
Ingredients : Avobenzone
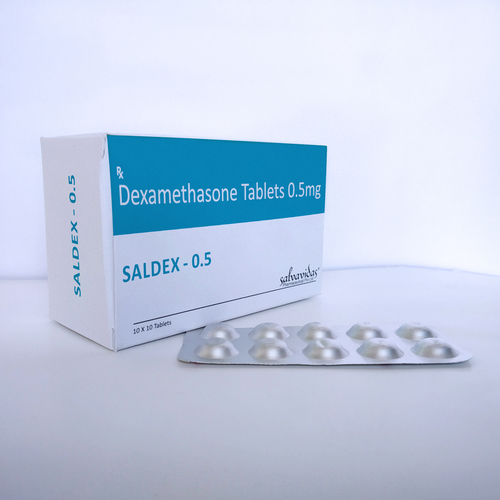
Dexamethasone Tablets
Storage Instructions : Store in Cool
Formulations Form : Tablets
Dosage Guidelines : Follow the dosage as advised by your healthcare provider
Formulations Type : General Drugs
Drug Type : Other, General Drugs
Ingredients : Dexamethasone
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry