

HYDROXYCHLOROQUINE SULFATE
HYDROXYCHLOROQUINE SULFATE Specification
- HS Code
- 29334990
- Structural Formula
- C18H26ClN3OH2SO4
- Smell
- Odorless
- Molecular Weight
- 433.95 g/mol
- EINECS No
- 212-017-2
- Color
- White
- Storage
- Store in a cool, dry place, protected from light
- Loss on Drying
- 0.5%
- Heavy Metal (%)
- 0.001%
- Solubility
- Freely soluble in water, soluble in alcohol
- Shelf Life
- 36 months
- Particle Size
- Micronized
- Melting Point
- 240-244C
- Poisonous
- Yes, toxic in high doses
- Taste
- Bitter
- Ph Level
- 4.5-7.0 (1% solution)
- Molecular Formula
- C18H26ClN3O.H2SO4
- Medicine Name
- HYDROXYCHLOROQUINE SULFATE
- Chemical Name
- HYDROXYCHLOROQUINE SULFATE
- CAS No
- 747-36-4
- Type
- API (Active Pharmaceutical Ingredient)
- Grade
- Pharmaceutical Grade
- Usage
- Used for the treatment of malaria, rheumatoid arthritis, and lupus erythematosus
- Purity(%)
- 99%
- Appearance
- White or almost white, crystalline powder
- Physical Form
- Solid
- Residual Solvents
- Complies with ICH guidelines
- pH (1% solution)
- 4.5-7.0
- Water Content
- 0.5%
- Assay
- 98.5% to 101.0%
- Specific Optical Rotation
- +79 to +85
- Identification
- By IR, complies with standard
- Related Substances
- 0.5%
HYDROXYCHLOROQUINE SULFATE Trade Information
- FOB Port
- All Indian Port
- Payment Terms
- Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Western Union, Paypal, Cash in Advance (CID)
- Supply Ability
- 50 Ton Per Month
- Delivery Time
- 7-10 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- As Per Buyer Requirements
- Main Export Market(s)
- Australia, South America, Western Europe, Middle East, Central America, Africa, Asia, Eastern Europe, North America
- Certifications
- ISO, GMP, WHO, MSDS
About HYDROXYCHLOROQUINE SULFATE
Product Name: HYDROXY CHLOROQUINE SULFATE
Grade: IP/BP/USP
Hydroxychloroquine is an antimalarial medicine which is used in the treatment and suppression of malaria. It is also used in the treatment of Rheumatoid Arthritis and Systemic Lupus Erythematosus.Close monitoring of blood glucose levels and muscle function is necessary while receiving the medicine.
Product Specifications
- Shelf Life
- 5 Years
- Molecular Formula
- C18H26ClN3O,H2SO4
- Molecular Weight
- 434.0 gm/mol
- Storage
- Keep away from moisture
- Chemical Name
- HYDROXY CHLOROQUINE SULFATE
- CAS No
- 747-36-4
- Type
- Other
- Grade
- Cosmetic Grade
- Usage
- Used in the treatment and suppression of malaria.
- Appearance
- crystalline solid
- Physical Form
- Powder
Quality and Compliance Standards
Hydroxychloroquine sulfate meets international pharmaceutical standards, with high assay values (98.5% to 101.0%) and minimal impurities. Rigorous testing ensures compliance with limits for related substances, water content, heavy metals, and residual solvents, making it suitable for safe medicinal formulation.
Versatility in Medical Applications
This API serves crucial roles in treating diseases like malaria, rheumatoid arthritis, and lupus erythematosus. Its effectiveness and broad application make it a staple raw material for medicine manufacturers, benefiting patients through improved therapeutic options.
Proper Handling and Storage
To preserve quality and potency, hydroxychloroquine sulfate should be stored in a dry, cool environment, protected from light. Its micronized powder form is easily integrated into various pharmaceutical formulations, but care should be taken due to its toxic potential at high doses.
FAQs of HYDROXYCHLOROQUINE SULFATE:
Q: How should hydroxychloroquine sulfate be stored to maintain its stability?
A: Store hydroxychloroquine sulfate in a cool, dry place, protected from light, to preserve its quality and extend shelf life. This prevents degradation and ensures efficacy over its 36 months shelf life.Q: What is the recommended usage of hydroxychloroquine sulfate?
A: Hydroxychloroquine sulfate is used as an active pharmaceutical ingredient for the treatment of malaria, rheumatoid arthritis, and lupus erythematosus. Dosage should be determined by a healthcare professional based on clinical needs.Q: When is hydroxychloroquine sulfate considered compliant for pharmaceutical use?
A: It is considered compliant when it meets specifications for assay (98.5%101.0%), related substances (0.5%), water content (0.5%), pH (4.57.0), and ICH guidelines for residual solvents, along with other established quality criteria.Q: Where is hydroxychloroquine sulfate manufactured and commonly exported from?
A: Hydroxychloroquine sulfate is manufactured and exported from India, supplied by certified exporters, manufacturers, and suppliers who follow global standards.Q: What process is used to identify and assure the quality of hydroxychloroquine sulfate?
A: Quality is confirmed through infrared (IR) identification, specific optical rotation checks (+79 to +85), and detailed analytical testing for purity, pH, water content, and residual solvents.Q: What are the benefits of using hydroxychloroquine sulfate in formulations?
A: Its high purity and compliance with global standards ensure safety and effectiveness in medical applications, offering reliable therapeutic value for patients suffering from malaria and autoimmune diseases.Q: Is hydroxychloroquine sulfate toxic, and what precautions are necessary?
A: Yes, hydroxychloroquine sulfate is toxic in high doses and should be handled with care. Only trained professionals should use it, adhering strictly to prescribed dosages and safety protocols.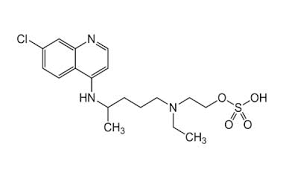

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Active Pharmaceutical Ingredients Category
FERRIC GLYCEROPHOSPHATE
Shelf Life : 36 months
Particle Size : Fine Powder
Solubility : Practically insoluble in water, soluble in dilute acids
Purity(%) : 98% min
Loss on Drying : Max 8.0%
Heavy Metal (%) : Max 0.002%
CLIOQUINOL
Shelf Life : 5 Years
Particle Size : As per requirement
Solubility : Slightly soluble in water, freely soluble in alcohol and ether
Purity(%) : 99% Min
Loss on Drying : Maximum 0.5%
Heavy Metal (%) : < 0.001%
FENBENDAZOLE
Shelf Life : 3 years
Particle Size : Micronized
Solubility : Practically insoluble in water
Purity(%) : 99%
Loss on Drying : 0.5%
Heavy Metal (%) : 0.002%
Niacin
Shelf Life : 3 years
Particle Size : 80 mesh
Solubility : Slightly soluble in water and ethanol
Purity(%) : 99%
Loss on Drying : Less than 0.5%
Heavy Metal (%) : Less than 0.002%
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry








