Survanta Suspension
Price 2-5 USD ($)/ Box
Survanta Suspension Specification
- Molecular Formula
- Complex mixture of phospholipids, neutral lipids, fatty acids, and surfactant proteins
- CAS No
- Not Applicable (Mixture)
- Purity
- 99%
- Application
- Hospital, Clinical
- Grade
- Pharmaceutical Grade
- Formulations Type
- Drug Solutions
- Formulations Form
- Liquid
- Treatments & Functions
- Prevention and treatment of Respiratory Distress Syndrome (RDS) in premature infants
- Gender/Age Group
- Adult and Pediatric
- Dosage Guidelines
- As Per Prescription
- Volume
- 8 ml Bottle
- Storage Instructions
- Dry Place
Survanta Suspension Trade Information
- Minimum Order Quantity
- 500 Boxes
- FOB Port
- Mundra Port
- Payment Terms
- Letter of Credit (L/C), Western Union, Paypal, Letter of Credit at Sight (Sight L/C), Telegraphic Transfer (T/T), Cash in Advance (CID), Cash Advance (CA)
- Supply Ability
- 10000 Boxes Per Month
- Delivery Time
- 30 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- As Per Buyer Requirements
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Main Domestic Market
- All India
- Certifications
- ISO, WHO, GMP, FDA
About Survanta Suspension
Survanta Suspension
Product Specifications
| Drug Type | Generic Drugs |
| Physical Form | Liquid |
| Dosage Guidelines | Consult your Doctor |
| Suitable For | Suitable For All |
Product Description
We have gained immense appreciation from clients for exporting an excellent stock of Survanta 8ml (Beractant Intratracheal). The medication is intended for intratracheal use only. This Survanta 8ml (Beractant Intratracheal) is indicated for the treatment of respiratory distress syndrome (RDS), also known as hyaline membrane disease, in newborn premature infants. We make sure to procure this medicine from the trusted and renowned vendors of the market.
Therapeutic indications
Survanta is indicated for treatment of Respiratory Distress Syndrome (RDS) (hyaline membrane disease) in new born premature infants with a birth weight of 700g or greater and who are intubated and are receiving mechanical ventilation.Survanta is also indicated for the prophylactic treatment of premature infants <32 weeks gestational age at risk of developing RDS.
Key points:
- It is a sterile and non-pyrogenic pulmonary surfactant
- The medication is a natural bovine lung extract
- It contains phospholipids, neutral lipids, fatty acids and surfactant-associated protein
Specifications:
- Brand: Survanta Susp
- Contain: Beractant Intratracheal
- Strength :4ml / 8ml
- Packing: Susp
Pharmacodynamic properties
Pharmacotherapeutic group: Lung Surfactant
- The mode of action of Survanta is biophysical rather than biochemical, i.e. it reduces surface tension and concomitantly increases lung compliance.
- Intratracheally administered Survanta distributes rapidly to the alveolar surfaces and stabilises the alveoli against collapse during respiration thereby increasing alveolar ventilation.
- In clinical studies of premature infants with Respiratory Distress Syndrome (RDS), a significant improvement in oxygenation was demonstrated after treatment with a single dose of Survanta.
- These infants showed a decreased need for supplemental oxygen and an increase in the arterial/alveolar oxygen ratio (a/Ap02). Significantly decreased need for respiratory support, as indicated by a lower mean airway pressure, was also observed. In most cases these effects were maintained for at least 72 hours after the administration of the single dose of Survanta.
Pharmacokinetic properties
In preclinical studies using radiolabelled phosphatidylcholine, the clearance rate of Survanta in the lung of three day old rabbits has been shown to be similar to that of natural calf and sheep surfactants (approximately 13% within 24 hours). In addition some re-uptake and secretion of Survanta was shown, implying its entry into a metabolically active surfactant pool.
Since an exogenous preparation of Survanta is delivered directly to the lung, classical clinical pharmocokinetic parameters (blood levels, plasma half-life etc.) have not been studied.
Preclinical safety data
There are no pre-clinical data of relevance to the prescriber which are additional to that already included in other sections of the SPC.
Special precautions for disposal and another handling
Each vial of Survanta is for single use only. Used vials with the residual drug should be discarded.Survanta should be inspected visually for discoloration prior to administration. The colour of Survanta is off-white to light brown. Some settling may occur during storage. If this occurs, gently invert the vial several times (DO NOT SHAKE) to redisperse.Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Effective RDS Management
Survanta Suspension is widely trusted in hospital and clinical settings for its efficacy in managing and preventing Respiratory Distress Syndrome (RDS) among premature infants. Its pharmaceutical-grade purity and precise formulation help stabilize lung function and improve neonatal outcomes.
Formulation and Safety
The unique blend of phospholipids, fatty acids, and surfactant proteins in Survanta Suspension mirrors natural lung surfactant, enhancing safety and compatibility. Each batch is rigorously tested, ensuring quality, purity, and reliability for sensitive patient populations.
FAQs of Survanta Suspension:
Q: How is Survanta Suspension administered?
A: Survanta Suspension is administered directly into the trachea (intratracheally) by a trained healthcare provider in a hospital or clinical setting. It is not for oral or intravenous use.Q: What conditions does Survanta Suspension treat?
A: Survanta Suspension is specifically indicated for the prevention and treatment of Respiratory Distress Syndrome (RDS) in premature infants, helping their lungs to work properly.Q: When should Survanta Suspension be used?
A: Survanta Suspension is used shortly after birth in premature infants at risk of RDS, or when RDS is diagnosed to support breathing and improve oxygen levels. The timing and dosing are determined by a physician.Q: Where should Survanta Suspension be stored?
A: Store Survanta Suspension in a dry place, away from direct sunlight and moisture, as recommended to maintain its stability and effectiveness.Q: What is the process for administering Survanta Suspension?
A: The administration involves a medical professional delivering the liquid into the infants lungs via the airway under controlled conditions in a healthcare environment, adhering to prescribed dosage guidelines.Q: What are the benefits of using Survanta Suspension?
A: The primary benefit is its ability to reduce the severity and incidence of RDS in premature infants, promoting better lung function and improved chances of survival.Q: Is a prescription required for Survanta Suspension?
A: Yes, Survanta Suspension is a prescription-only medication and should only be used under the supervision of qualified medical personnel.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Critical Care Products Category
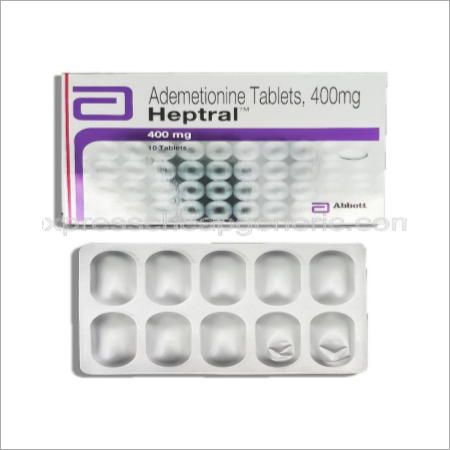
Ademetionine Tablets
Price 2-3 USD ($)
Minimum Order Quantity : 500 Boxes
Formulations Form : Tablets
Formulations Type : General Drugs
Dosage Guidelines : As directed by physician
Storage Instructions : Dry Place
Levocetirizine Dihydrochloride Tablet
Price 0.10-0.15 USD ($)
Minimum Order Quantity : 500 Boxes
Formulations Form : Tablets
Formulations Type : General Drugs
Dosage Guidelines : As per physicians direction
Storage Instructions : Dry Place
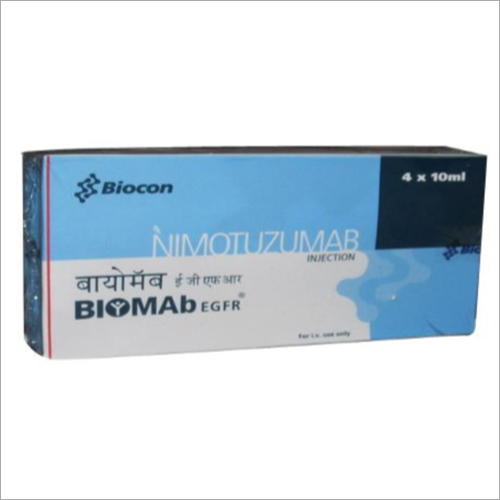
Nimotuzumab Injection
Price 100-200 USD ($)
Minimum Order Quantity : 10 Boxes
Formulations Form : Liquid
Formulations Type : Injectables
Dosage Guidelines : As Directed by Physician
Storage Instructions : Store in Cool
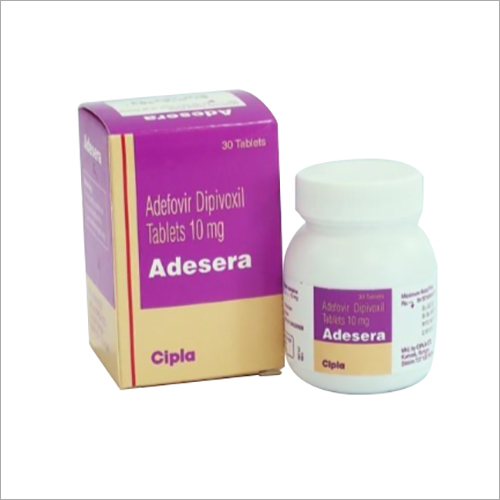
Adefovir Dipivoxil Tablets
Price 2-3 USD ($)
Minimum Order Quantity : 100 Boxes
Formulations Form : Tablets
Formulations Type : General Drugs
Dosage Guidelines : As directed by physician
Storage Instructions : Dry Place
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry