Pantoprazole with Domperidone Capsules
Pantoprazole with Domperidone Capsules Trade Information
- Minimum Order Quantity
- 100 Boxes
- FOB Port
- Mundra Port
- Supply Ability
- 100000 Boxes Per Month
- Delivery Time
- 30 Days
- Sample Policy
- Within a certain price range free samples are available
- Main Domestic Market
- All India
- Certifications
- ISO, GMP, WHO
About Pantoprazole with Domperidone Capsules
Pantoprazole with Domperidone Capsules
The combination of domperidone and pantoprazole is marketed by Rewine Pharmaceuticals.
Pantoprazole acts by selectively inhibiting the H+/K+-ATPase enzyme in the secretory canaliculus of the stimulated parietal cell. Domperidone stimulates GI activity by acting as a competitive antagonist at dopamine D2-receptors.
Pantoprazole: Diarrhoea, dizziness, pruritus, skin rashes, GIT infections; anaphylaxis, angioedema, chest pain, dyspnoea, erythema multiforme, gastroenteritis, hyperglycaemia, infection, Inj site reaction, jaundice, optic neuropathy, anterior ischaemia, pancreatitis, speech disorder. Domperidone: Headache, insomnia, nervousness, dizziness, thirst, lethargy, irritability, GI disturbances, hot flushes, mastalgia, galactorrhoea, gynaecomastia, menstrual irregularities, rash, pruritus, urticaria, stomatitis, conjunctivitis, urinary frequency, dysuria, oedema, palpitations, leg cramps, asthaenia, drug intolerance.
Lactation. Pantoprazole: Not recommended in child <12 years. Long-term therapy may lead to bacterial overgrowth in the GIT. Domperidone: Increases serum prolactin levels resulting to galactorrhoea in females and gynaecomastia in males. Hypertensive crisis in patients with phaeochromocytoma.
Care should be exercised when domperidone is administered in combination with MAO inhibitors.
Oral
Gastro-oesophageal reflux disease, Dyspepsia
Adult: Per tablet/capsule contains pantoprazole 40 mg and domperidone 10 mg: 1 tablet/capsule once daily.
Dual-Action Relief for Digestive Discomfort
Pantoprazole with Domperidone Capsules offer a synergistic approach to gastrointestinal issues. Pantoprazole reduces acid production in the stomach, while Domperidone promotes healthy movement of food through the digestive tract. This combination addresses both the symptoms and underlying causes of acidity and ulcers for comprehensive relief.
Easy Administration and Storage
These capsules are designed for convenient oral administration. Patients are advised to take the medication as directed by their healthcare provider. For optimal effectiveness and longevity, the capsules should be kept in a dry place, away from moisture and direct sunlight.
Assured Quality from Trusted Manufacturers
Produced in India by experienced exporters, manufacturers, and suppliers, these capsules meet stringent quality benchmarks. The product supports global demand for reliable, general pharmaceutical solutions and is backed by robust manufacturing processes.
FAQs of Pantoprazole with Domperidone Capsules:
Q: How do Pantoprazole with Domperidone Capsules help in treating acidity and heartburn?
A: Pantoprazole reduces gastric acid production, while Domperidone enhances gastrointestinal motility. Together, they provide dual relief from symptoms like acidity, heartburn, and stomach ulcers.Q: What is the recommended way to take these capsules?
A: These capsules should be taken orally with water, preferably before meals, as advised by a healthcare provider for maximum effectiveness.Q: When is the best time to use Pantoprazole with Domperidone Capsules?
A: It is generally recommended to take the capsules before meals, once daily or as directed by your physician, to manage and prevent acidity and discomfort.Q: Where should I store these capsules for best results?
A: Pantoprazole with Domperidone Capsules should be stored in a dry place, away from heat and moisture, to preserve their potency and shelf-life.Q: What are the main benefits of using this combination formulation?
A: This combination provides rapid relief from acid-related discomfort, helps heal stomach ulcers, and improves digestive function with a single, convenient capsule.Q: How are these capsules manufactured and supplied from India?
A: Produced by reputed Indian exporters, manufacturers, and suppliers, the capsules undergo strict quality checks before global distribution to ensure safety and efficacy.Q: What is the process for obtaining these capsules from an Indian supplier?
A: Interested buyers can contact certified exporters, manufacturers, or suppliers in India to place bulk orders, ensuring compliance with regulatory and quality requirements.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Anti-Ulcer Medicines Category
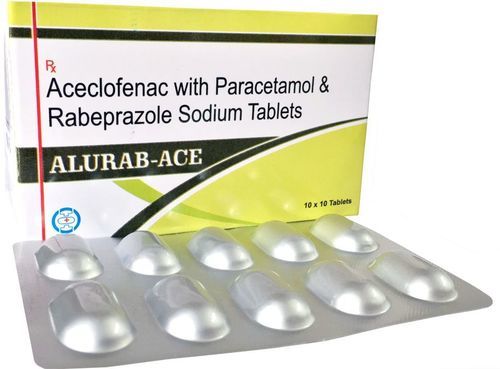
Aceclofenac, Paracetamol and Rabeprazole Tablet
Price 0.1-0.3 USD ($)
Minimum Order Quantity : 100 Boxes
Ingredients : Aceclofenac, Paracetamol, Rabeprazole
Suitable For : Adults
Formulations Form : Tablets
Salt Composition : Aceclofenac 100mg, Paracetamol 325mg, Rabeprazole 20mg
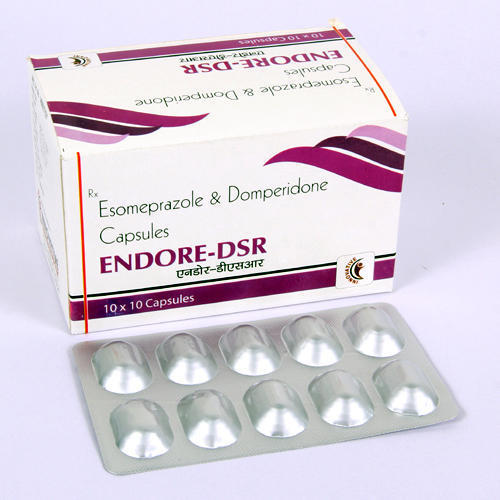
Esomeprazole and Domperidone Capsules
Price 0.1-0.2 USD ($)
Minimum Order Quantity : 100 Boxes
Ingredients : Esomeprazole and Domperidone
Suitable For : Adults
Formulations Form : Capsules
Salt Composition : Esomeprazole 40 mg + Domperidone 30 mg
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry