Methylprednisolone Succinate Injection
Methylprednisolone Succinate Injection Specification
- Formulations Type
- Injectables
- Formulations Form
- Liquid
- Gender/Age Group
- Adult, Women
- Storage Instructions
- Store in Cool
Methylprednisolone Succinate Injection Trade Information
- FOB Port
- All Indian Port
- Payment Terms
- Letter of Credit (L/C), Telegraphic Transfer (T/T), Paypal, Western Union
- Supply Ability
- 100000 Piece Per Month
- Delivery Time
- 7-10 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- As Per Buyer Requirements
- Main Export Market(s)
- Australia, North America, South America, Eastern Europe, Middle East, Africa, Central America, Western Europe, Asia
- Main Domestic Market
- All India
- Certifications
- ISO, GMP, WHO, FDA, MFG. License
About Methylprednisolone Succinate Injection
We are a leading Manufacturer and Exporter of Methyl Prednisolone Succinate Injection in different strength and packing. Our manufacturing facility is certified with WHO-GMP.
Methyl Prednisolone Sodium Succinate 40/125/500/1000mg in injection Promotes Methyl Parednisolone Sodium Succinate 40/125/500/1000mg in injection against neurological disease and aging relieves burning sensations, numbness, loss of sensation, mudcle cramps in diabetic neuropathy exhibits anxiolyric, analgesic and anti convulsant activiry2.5 time more potent then gabapent in based on plasma concentrations provides early sustained improvement in pain and a beneficial effect on sleep on diabetic.
Composition :
Methyl Prednisolone Succinate 125 Mg
Methyl Prednisolone Succinate 500 Mg
Methyl Prednisolone Succinate 1 Gm
Specifications :
- Product Type : Finished Product
- Dose/Strength (ex. 1 mg or 1 ml) : 40/125/500/1000
- Size : 1*1
- Usage : Clinical,Hospital
We manufacture and export our brand.We also offer private labeling, Contract manufacturing service.
Reliable Injectable Solution
Our Methylprednisolone Succinate Injection is meticulously formulated in a liquid form to ensure reliable, immediate absorption. Specially tailored for adult and female patients, this injectable is an effective option for managing acute inflammation and immune-mediated conditions. Precision manufacturing standards and quality assurance protocols make it a trusted choice for healthcare institutions.
Stringent Storage and Handling
Proper storage is vital for maintaining the potency and safety of Methylprednisolone Succinate Injection. We recommend storing the product in a cool location as indicated on the packaging. Following these guidelines will preserve the formulations effectiveness and stability, guaranteeing the best patient outcomes.
FAQs of Methylprednisolone Succinate Injection:
Q: How should Methylprednisolone Succinate Injection be stored?
A: Methylprednisolone Succinate Injection should be stored in a cool environment, as excessive heat or improper storage can compromise its effectiveness. Always refer to the product label for detailed storage instructions.Q: What are the main uses of this injection?
A: This injectable formulation is primarily used to manage moderate to severe inflammatory and autoimmune conditions in adults, including women. It is favored for its quick onset of action in urgent medical situations.Q: When is Methylprednisolone Succinate Injection typically administered?
A: The injection is generally administered during acute flare-ups of inflammatory diseases, or when oral corticosteroids are insufficient or impractical. A healthcare professional will decide the appropriate timing and dosage.Q: Where is this product typically used?
A: Methylprednisolone Succinate Injection is commonly administered in hospitals, clinics, or other healthcare facilities where trained personnel can ensure accurate dosing and monitor for adverse effects.Q: What is the process for manufacturing and exporting this injection from India?
A: Manufacturing follows strict quality and safety guidelines in GMP-certified facilities. After meeting regulatory standards, the product is supplied to both domestic and international markets by trusted Indian exporters and suppliers.Q: How is the medication administered and by whom?
A: This medication is administered via injection by a qualified healthcare professional. Self-administration is not recommended due to the need for precise dosing and monitoring.Q: What are the benefits of using the injectable form over oral corticosteroids?
A: The injectable form offers a more rapid onset of action, which is beneficial during acute or severe episodes. It is also advantageous when patients are unable to take oral medications.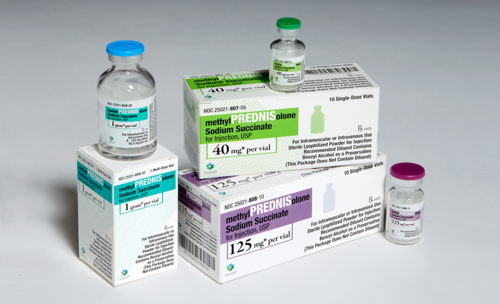

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Critical Care Products Category
Pramipexole Di-hydrochloride monohydrate Tablet
Price 1-5 USD ($)
Minimum Order Quantity : 500 Boxes
Formulations Form : Tablets
Dosage Guidelines : As Directed By Physician
Application : Parkinsons Disease Treatment
Formulations Type : General Drugs
Levocetirizine Dihydrochloride Tablet
Price 0.10-0.15 USD ($)
Minimum Order Quantity : 500 Boxes
Formulations Form : Tablets
Dosage Guidelines : As per physicians direction
Application : Antiallergic
Formulations Type : General Drugs
Lamivudine, Stavudine and Nevirapine Tablets
Price 6-7 USD ($)
Minimum Order Quantity : 500 Boxes
Formulations Form : Tablets
Dosage Guidelines : As per physicians direction
Application : AntiHIV Drugs
Formulations Type : Drug Mixture
Tenofovir, lamivudine and Efavirenz Tablet
Price 10-20 USD ($)
Minimum Order Quantity : 100 Boxes
Formulations Form : Tablets
Dosage Guidelines : As directed by physician
Application : Antiviral, HIV treatment
Formulations Type : General Drugs
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry