Gefitinib Tablet
Gefitinib Tablet Specification
- Indication
- Non-Small Cell Lung Cancer (NSCLC)
- Salt Composition
- Gefitinib 250 mg
- Dosage Form
- Tablet
- Feature
- Oral, easy-to-administer, targeted therapy
- Ingredients
- Gefitinib
- Application
- Anti-cancer, targeted therapy
- Physical Color/Texture
- White to off-white film-coated tablets
- Shelf Life
- 24 months
Gefitinib Tablet Trade Information
- Minimum Order Quantity
- 100 box,
- Payment Terms
- Letter of Credit (L/C), Paypal, Western Union, Cash in Advance (CID), Cash Advance (CA)
- Delivery Time
- 30 Days
- Sample Policy
- Within a certain price range free samples are available
- Main Export Market(s)
- Australia, North America, Eastern Europe, Middle East, Western Europe, Africa, Central America, South America, Asia
- Certifications
- ISO, GMP, WHO
About Gefitinib Tablet
Product Description
- We Rewine Pharmaceutical are a leading Manufacturer and Exporter of Gefitinib Tablet in different strength and packing. Our manufacturing facility is certified with WHO-GMP.
- Geftinat contains gefitinib and belongs to a class of called epidermal growth factor receptor (egfr) tyrosine kinase inhibitors. Geftinat is used for the treatment of non-small cell lung .
Composition: Gefitinib 250 mg Tablet
Specifications:
- Brand Name : Gefitinib
- Generic Name : Gefitinib
- Company : Astra Zeneca
- Strength : 250mg
- Packing : 3x10
- Type : Tablets
- Product Type : Finished Product
- Usage: Clinical,Hospit
Targeted Therapy for NSCLC
Gefitinib Tablets are designed to provide targeted treatment for patients diagnosed with Non-Small Cell Lung Cancer (NSCLC). By specifically inhibiting key growth receptors in cancer cells, this oral medication helps halt the progression of disease and offers a tailored approach compared to traditional chemotherapy.
Convenient Oral Administration
These tablets are meant for easy and consistent oral administration, making them straightforward for patients to use at home. Each strip contains 10 film-coated tablets, simplifying dosage management and ensuring compliance with prescribed therapy.
Quality, Stability, and Shelf Life
Gefitinib Tablets have a shelf life of 24 months when stored correctly in a cool and dry environment. The robust packaging and film-coating preserve tablet efficacy, allowing you to maintain the medications quality throughout its intended duration.
FAQs of Gefitinib Tablet:
Q: How should Gefitinib Tablets be administered for NSCLC?
A: Gefitinib Tablets are taken orally, typically once daily at a fixed dose of 250 mg per tablet, as directed by a physician. The tablet can be taken with or without food, but consistent timing enhances efficacy.Q: What is the recommended storage condition for Gefitinib Tablets?
A: Store Gefitinib Tablets in a cool and dry place, away from direct sunlight and moisture, to preserve their potency. Avoid keeping them in humid environments such as bathrooms.Q: When is Gefitinib indicated for patient therapy?
A: Gefitinib is prescribed for adult patients diagnosed with Non-Small Cell Lung Cancer (NSCLC), particularly as a targeted therapy in cases where specific genetic mutations are detected. A physician will determine suitability based on clinical evaluation.Q: Where does one obtain Gefitinib Tablets, and is a prescription necessary?
A: Gefitinib Tablets are available from authorized exporters, manufacturers, and suppliers in India. A physicians prescription is required, as this is not an over-the-counter medication due to its potent, targeted nature.Q: What is the main benefit of using Gefitinib Tablets in NSCLC treatment?
A: The primary benefit is targeted inhibition of cancer cell growth with lower impact on normal tissues, which reduces some side effects compared to conventional chemotherapy and provides tailored therapy for eligible patients.Q: How long can Gefitinib Tablets be stored before they expire?
A: Gefitinib Tablets have a shelf life of 24 months from the date of manufacture, provided they are stored in the recommended conditions.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Oncology Drugs Category
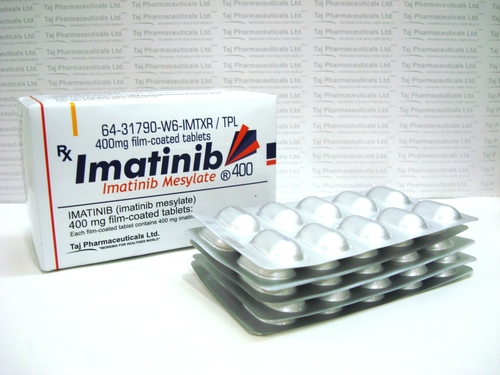
Imatinib Mesylate Tablets
Price 8-10 USD ($)
Minimum Order Quantity : 100 Boxes
Physical Color/Texture : Other , Yellowish to brownish colored round tablets
Feature : Other, Film Coated, Oral Administration
Shelf Life : 24 months
Application : Other, Cancer Treatment
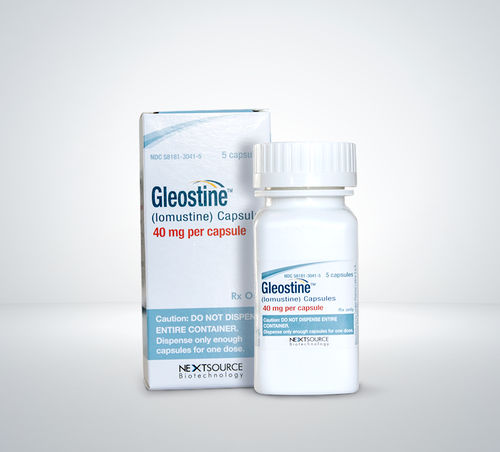
Lomustine Capsule
Price 8-9 USD ($)
Minimum Order Quantity : 100 Boxes
Physical Color/Texture : Other , Yellow opaque capsules
Feature : Other, Oral chemotherapy capsule, easy administration
Shelf Life : 24 months
Application : Other, Antineoplastic (chemotherapy) agent
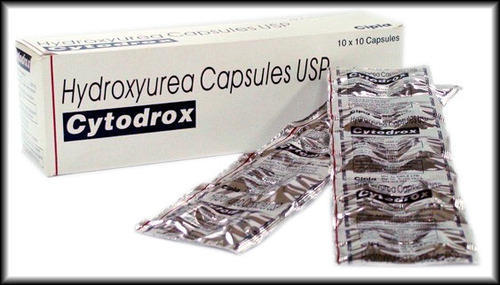
Hydroxyurea Capsule
Price 8-9 USD ($)
Minimum Order Quantity : 100 Boxes
Physical Color/Texture : Other , Opaque white capsules
Feature : Other, Oral chemotherapy, Capsule form
Shelf Life : 24 months
Application : Other, Antineoplastic, Sickle cell disease management
Lapatinib Tablet
Price 25-50 USD ($)
Minimum Order Quantity : 50 Boxes
Physical Color/Texture : Other , Yellow colored filmcoated tablet
Feature : Other, Oral administration, targeted therapy
Shelf Life : 2 years from date of manufacture
Application : Other, Oncology
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry