Flurouracil Injection
Flurouracil Injection Specification
- Dosage Form
- Injection
- Salt Composition
- Fluorouracil 250 mg/mL
- Indication
- Antineoplastic (used in the treatment of cancer such as colorectal, breast, stomach, and pancreatic cancers)
- Feature
- Sterile, ready-to-use, high purity, non-pyrogenic
- Ingredients
- Fluorouracil, Water for Injection
- Application
- Ph Level
- Approximately 9.2
- Physical Color/Texture
- Clear, colorless solution
- Fermentation Smell
- Odorless
- Shelf Life
- 36 months
Flurouracil Injection Trade Information
- Minimum Order Quantity
- 100 Piece
- Payment Terms
- Letter of Credit (L/C), Western Union, Paypal, Cash in Advance (CID), Cash Advance (CA)
- Delivery Time
- 30 Days
- Sample Policy
- Within a certain price range free samples are available
- Main Export Market(s)
- Australia, Eastern Europe, Africa, Central America, Middle East, South America, Western Europe, Asia, North America
- Certifications
- ISO, GMP, WHO
About Flurouracil Injection
Flurouracil Injection
Product Details:
- Dose: 250/500 mg
- Usage: Clinical
- Packaging Size: 1*1
- Packaging Type: Vial
We Rewine Pharmaceutical are a leading Manufacturer and Exporter of Flurouracil injection in different strength and packing. Our manufacturing facility is certified with WHO-GMP.
Composition ::
- Flurouracil 250 MG injection
- Flurouracil 500 MG injection
- Product Type : Finished Product
- Dose/Strength (ex. 1 mg or 1 ml): 250/500 MG
- Type : Vial
- Packing : 1*1
- Usage: Clinical, Hospital
- High protein binding ability
- Accurate composition
- Optimum purity
USP Grade Antineoplastic Efficacy
Fluorouracil Injection meets stringent USP standards, ensuring a high level of purity and reliable therapeutic effect in the management of cancers. This medicine is used intravenously or intraarterially by healthcare professionals, offering effective action against various solid tumors.
Sterile, Ready-to-Use Convenience
The injection is formulated as a liquid in a sterile, non-pyrogenic presentation and is available in multiple vial sizes for flexible dosing. It is ready for direct use without the need for reconstitution, streamlining the preparation process in clinical environments.
Safety and Handling Precautions
Due to its cytotoxic properties, Fluorouracil Injection requires careful handling with appropriate personal protective equipment to safeguard healthcare providers. Detailed guidelines on safe administration, disposal, and spill management support compliance and safety.
FAQs of Flurouracil Injection:
Q: How should Fluorouracil Injection be administered to patients?
A: Fluorouracil Injection is intended for intravenous or intraarterial administration by trained healthcare professionals. The dosage and infusion rate should be individualized based on the patients specific cancer type and treatment protocol.Q: What cancers can be treated with Fluorouracil Injection?
A: This injection is primarily prescribed for the treatment of colorectal, breast, stomach, and pancreatic cancers. Its antineoplastic properties make it a key agent in many chemotherapy regimens.Q: When is it necessary to use cytotoxic precautions with this product?
A: Cytotoxic precautions must always be used during handling, preparation, administration, and disposal since Fluorouracil Injection poses risks of cytotoxic exposure to healthcare providers. Personal protective equipment is strongly recommended.Q: Where should Fluorouracil Injection be stored for optimal stability?
A: The vials should be stored in a cool environment as outlined in the products storage instructions, away from direct heat and light to maintain stability and efficacy for up to 36 months.Q: What makes this formulation compatible with standard I.V. fluids?
A: Fluorouracil Injection is USP-grade and specifically formulated to mix safely with most conventional I.V. fluids, allowing for flexibility in clinical administration and reducing the risk of precipitation or incompatibility.Q: How does Fluorouracil Injection benefit patient treatment?
A: As a high-purity, sterile, and ready-to-use antineoplastic injection, this product delivers targeted cancer therapy, supporting improved patient outcomes under professional supervision in a hospital setting.Q: What is the physical appearance and scent of Fluorouracil Injection?
A: The solution is clear, colorless, and free of odor, which helps confirm its purity and quality for patient safety during administration.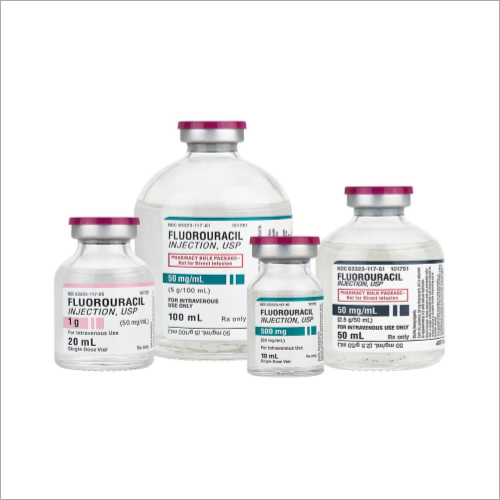
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Oncology Drugs Category
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry