Methotrexate Injection
Methotrexate Injection Specification
- Indication
- Antineoplastic, Antirheumatic (used for treatment of certain cancers, rheumatoid arthritis, and psoriasis)
- Dosage Form
- Injection
- Salt Composition
- Methotrexate Sodium
- Feature
- Sterile, Ready-to-use, Preservative-free
- Ingredients
- Methotrexate Sodium, Water for Injection, Preservatives (if applicable)
- Application
- Intramuscular, Intravenous, Subcutaneous or Intra-arterial injection as per physicians direction
- Ph Level
- 7.0 8.5
- Temperature Needed For Fermentation
- Not Applicable (No fermentation process)
- Physical Color/Texture
- Clear, Yellowish Solution
- Fermentation Smell
- Odorless (No fermentation)
- Storage Instructions
- Store below 25C. Protect from light. Do not freeze.
- Shelf Life
- 24 months from manufactured date
Methotrexate Injection Trade Information
- FOB Port
- All Indian Port
- Payment Terms
- Western Union, Paypal, Telegraphic Transfer (T/T), Cash in Advance (CID)
- Delivery Time
- 7 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- As Per Standard Packing
- Main Export Market(s)
- Western Europe, Australia, North America, Eastern Europe, Middle East, Africa, Central America, South America, Asia
- Certifications
- ISO, GMP, WHO
About Methotrexate Injection
Methotrexate Injection
- Product Name : Methotrexate Injection
- Trade Name : Trexall, Rheumatrex, Otrexup, Methotex
- Available Strength : 50mg, 500mg, 1gm.
- Packing : Sterile Single Dose vial
- Pack Insert/Leaflet : Yes
- Therapeutic use : Anti Cancer
- Production Capacity : 10,000 inj./Month
What Is Methotrexate?
Methotrexate injection is an antineoplastic chemotherapy drug. It is classified as an antimetabolite and given in combination with other anticancer drugs or alone.
Methotrexate Injection Uses
This medication is used for the treatment of
- Brest cancer
- Gestational trophoblastic tumors
- Lung cancer
- Meningeal leukemia
- Acute lymphotic leukemia
- Non -Hodgkins lymphoma
- Osteosarcoma
How Does Fludarabine Work?
It works by slowing down the growth of the cancer cell making it stop their DNA repairing for growth and multiplication.
Available Strength:
- Methotrexate injection 50mg
- Methotrexate injection 500mg
- Methotrexate injection 1gm
Precise Pharmaceutical Formulation
Methotrexate Injection is formulated to meet rigorous USP standards for clarity and color, ensuring pharmaceutical reliability. It contains Methotrexate Sodium, Water for Injection, and, if required, preservatives. The solution is clear to yellowish, odorless, and free from particulates. Its pH level ranges between 7.0 and 8.5, supporting optimal stability and compatibility with normal saline and 5% dextrose.
Hospital Use and Administration
This injection is intended strictly for hospital use, administered under the supervision of qualified healthcare professionals. It accommodates multiple routes, including intramuscular, intravenous, subcutaneous, and intra-arterial injections, as per the treating physicians directions. The sterile, ready-to-use packaging supports immediate patient care and safety protocols.
Storage, Safety, and Regulatory Compliance
Methotrexate Injection must be stored below 25C, protected from light, and not frozen. Each unit displays batch and expiry details for traceability. Manufactured under GMP standards and DCGI approval, it is a cytotoxic agent requiring careful handling. Its shelf life is 24 months from the manufacturing date, ensuring long-term efficacy for hospital pharmacies.
FAQs of Methotrexate Injection:
Q: How should Methotrexate Injection be stored to maintain its potency?
A: Methotrexate Injection should be stored below 25C, protected from light, and must not be frozen. Adhering to these instructions helps ensure product stability and effectiveness over its 24-month shelf life.Q: What is the recommended process for administering Methotrexate Injection?
A: Methotrexate Injection must be administered by a qualified healthcare professional in a hospital setting. The routeintramuscular, intravenous, subcutaneous, or intra-arterialis determined by the physician based on the clinical needs of the patient.Q: When is Methotrexate Injection indicated for use?
A: This medication is indicated for the treatment of certain cancers, rheumatoid arthritis, and psoriasis. The specific dosage and administration depend on the physicians assessment and patients condition.Q: Where can Methotrexate Injection be sourced and who manufactures it?
A: Methotrexate Injection is supplied by licensed exporters, manufacturers, and suppliers in India. Each product undergoes GMP-compliant manufacturing and carries DCGI approval for regulatory assurance.Q: What are the benefits of using Methotrexate Injection in clinical practice?
A: Methotrexate Injection offers reliable antineoplastic and antirheumatic effects, sterile and ready-to-use form, and compatibility with normal saline and 5% dextrose, making it effective and versatile across various therapeutic indications.Q: Is Methotrexate Injection compatible with other intravenous fluids?
A: Yes, the injection is compatible with normal saline and 5% dextrose, supporting flexible administration options for healthcare professionals.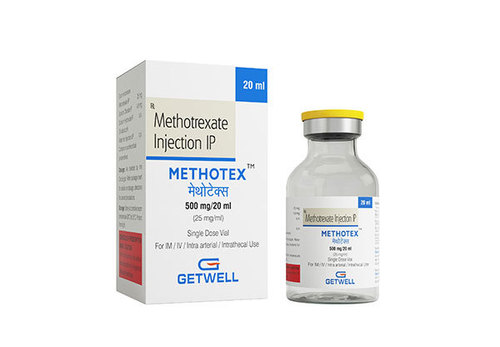

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Oncology Drugs Category
Carboplatin Injection
Price 5-10 USD ($)
Minimum Order Quantity : 100 Boxes
Feature : Other, Sterile, nonpyrogenic
Dosage Form : Injection
Indication : Antineoplastic; Treatment of ovarian, lung, and other cancers
Ingredients : Other , Carboplatin, water for injection, stabilizers
Lenalidomide Tablet
Price 9-10 USD ($)
Minimum Order Quantity : 100 Boxes
Feature : Other, Oral administration; easy to use
Dosage Form : Tablet
Indication : Multiple Myeloma, Myelodysplastic Syndromes
Ingredients : Other , Lenalidomide, Excipients
Anastrozole Tablet
Price 3500 INR
Minimum Order Quantity : 100 Boxes
Feature : Other, Highly potent, Selective nonsteroidal aromatase inhibitor
Dosage Form : Tablet
Indication : Used for treatment of breast cancer in postmenopausal women
Ingredients : Other , Anastrozole, Excipients
VALACYCLOVIR TABLET
Price 3-4 USD ($)
Minimum Order Quantity : 100 Boxes
Feature : Other, Highly Effective against Herpes Viruses
Dosage Form : Tablet
Indication : Herpes Zoster (Shingles), Genital Herpes, and Cold Sores
Ingredients : Other , Valacyclovir Hydrochloride and Excipients
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry