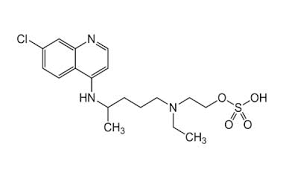
Dorzalamide HCl
Dorzalamide HCl Specification
- Storage
- Room Temperature
- Grade
- Medicine Grade
- Purity(%)
- 99
- Appearance
- white crystalline powder
- Physical Form
- Powder
Dorzalamide HCl Trade Information
- Minimum Order Quantity
- 10 Kilograms
- Supply Ability
- 100 Kilograms Per Week
- Delivery Time
- 7 Days
About Dorzalamide HCl
Product Details:|
Grade Standard |
Medicine Grade |
|
API Form |
Powder |
IUPAC Name:(4R,6R)-4-(ethylamino)-6-methyl-7,7-dioxo-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide hydrochloride
CAS Registry Number:130693-82-2
Consistent Quality for Pharmaceutical Use
Our Dorzalamide HCl is produced to exacting pharmaceutical-grade standards, ensuring a purity of 99% and a reliable white crystalline powder appearance. Each batch is thoroughly tested, making our product suitable for a variety of medical and research applications. The consistency in quality positions our Dorzalamide HCl as a preferred choice for medicine manufacturers and suppliers globally.
Secure Packaging and Easy Storage
We offer Dorzalamide HCl in secure packaging that maintains its integrity during transit and storage. Designed to be stable at room temperature, the products shelf life and physical properties are preserved, resulting in minimal loss or degradation. Our comprehensive storage guidelines help customers retain product quality over extended periods.
Proudly Exported from India
As a trusted manufacturer, exporter, and supplier in India, we consistently meet international standards. Dorzalamide HCl is shipped efficiently worldwide, ensuring timely delivery. Our robust logistics network and experienced export team facilitate hassle-free sourcing for clients in various pharmaceutical markets.
FAQs of Dorzalamide HCl:
Q: What is Dorzalamide HCl commonly used for?
A: Dorzalamide HCl is primarily utilized in the formulation of ophthalmic solutions to lower intraocular pressure in patients with glaucoma or ocular hypertension. Its high purity and pharmaceutical grade make it suitable for safe medical applications.Q: How should Dorzalamide HCl powder be stored?
A: Dorzalamide HCl should be stored at room temperature, away from excessive moisture and direct sunlight. Proper storage ensures its stability and preserves its white crystalline powder form for long-term use.Q: What is the process of manufacturing Dorzalamide HCl in your facility?
A: Our manufacturing process involves strict adherence to quality control protocols. Using advanced chemical synthesis techniques, we achieve 99% purity and a consistent crystalline structure, followed by thorough quality testing before packaging.Q: Where is your Dorzalamide HCl exported from?
A: We manufacture, supply, and export Dorzalamide HCl from India. Our facilities meet international standards, allowing us to serve customers worldwide with a reliable and high-quality product.Q: What are the benefits of using high-purity Dorzalamide HCl?
A: Using high-purity Dorzalamide HCl ensures better efficacy, safety, and consistency in pharmaceutical preparations. It minimizes the risk of impurities, which is critical for sensitive ophthalmic applications.Q: When should Dorzalamide HCl be considered for use in pharmaceutical products?
A: Dorzalamide HCl should be considered when formulating eye drops or other medications requiring the reduction of intraocular pressure. It is a preferred choice due to its proven effectiveness and high purity.Q: How is Dorzalamide HCl typically used in medical practice?
A: It is most commonly used as an active ingredient in eye drops prescribed for glaucoma or ocular hypertension. Medical professionals prescribe it to help manage and control elevated eye pressure.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Active Pharmaceutical Ingredients Category
FERROUS GLUCONATE
Shelf Life : 3 Years
Grade : Other, Pharmaceutical Grade
Storage : Other, Store in a cool, dry place, protected from light
Physical Form : Other, Solid Powder
Color : Other, Grayish green
Medicine Name : Ferrous Gluconate
HYDROXYCHLOROQUINE SULFATE
Shelf Life : 36 months
Grade : Other, Pharmaceutical Grade
Storage : Other, Store in a cool, dry place, protected from light
Physical Form : Solid
Color : White
Medicine Name : HYDROXYCHLOROQUINE SULFATE
FERRIC AMMONIUM CITRATE
Shelf Life : 3 Years
Grade : Other, Chemical Grade
Storage : Other, Store in a cool and dry place
Physical Form : Powder
Color : Other, Greenish yellow
Medicine Name : Ferric Ammonium Citrate
Roxithromycin
Shelf Life : 2 years
Grade : Other, Pharmaceutical Grade
Storage : Other, Store at room temperature in a tightly sealed container away from light and moisture
Physical Form : Solid
Color : White
Medicine Name : Roxithromycin
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry