Cytarabine Injection
Cytarabine Injection Specification
- Salt Composition
- Cytarabine
- Dosage Form
- Liquid Injection
- Indication
- Used in the treatment of certain cancers such as leukemia (including acute myeloid leukemia, acute lymphocytic leukemia, and chronic myelogenous leukemia) and lymphomas.
- Feature
- Sterile, pyrogen free, ready-to-use
- Ingredients
- Cytarabine and suitable excipients
- Application
- Intravenous or subcutaneous injection for oncology therapeutic use
- Ph Level
- Typically 7.0 - 8.5 (adjusted for injection preparation)
- Temperature Needed For Fermentation
- Not Applicable (Synthetic chemical preparation)
- Physical Color/Texture
- Clear, colorless to pale yellow liquid
- Fermentation Smell
- Odorless
- Shelf Life
- 24 months from manufacture date
- Manufacturing Method
- Aseptic manufacturing under GMP guidelines
- Strength
- Available as 100 mg/1 ml and 500 mg/5 ml vials
- Contraindications
- Hypersensitivity to Cytarabine or any component
- Compatibility
- Compatible with standard IV infusion fluids
- Prescription/Non Prescription
- Prescription
- Packaging Type
- Glass vial with flip-off seal
- Administration Route
- Intravenous (IV) or Subcutaneous (SC)
- USP/BP/EP Standards
- Meets pharmacopeial standards (USP/BP/EP)
Cytarabine Injection Trade Information
- Minimum Order Quantity
- 100 Piece
- Delivery Time
- 30 Days
- Sample Policy
- Within a certain price range free samples are available
- Certifications
- ISO, GMP, WHO
About Cytarabine Injection
Cytarabine Injection
Product Details:
| Dose | 100 mg/500mg/1gm |
| Usage | Clinical, Hospital, Personal |
| Packaging Size | 1*1 |
| Packaging Type | Vial |
We Rewine Pharmaceutical are a leading Manufacturer and Exporter of Cytarabine injection in different strength and packing. Our manufacturing facility is certified with WHO-GMP.
Cytarine Injection is a medicine that is used for the treatment of Cancers of the blood, Cancer of the lymph glands and other conditions. Cytarine Injection contains Cytosine Arabinoside as an active ingredient. Cytarine Injection works by inhibiting the synthesis of DNA.
Composition :
- Cytarabine 100mg
- Cytarabine 500mg
- Cytarabine 1gm
Product Type : Finished Product
- Dose/Strength (ex. 1 mg or 1 ml): 100 mg/500mg/1gm
- Type : Vial
- Packing : 1*1
- Usage: Clinical, Hospital
We manufacture and export our brand. We also offer private labelling, Contract manufacturing service.
Effective Treatment for Leukemia and Lymphoma
Cytarabine Injection is widely used as a key chemotherapeutic agent for managing leukemias such as acute myeloid leukemia (AML), acute lymphocytic leukemia (ALL), and chronic myelogenous leukemia (CML), as well as lymphoma. Its targeted mechanism helps inhibit abnormal cell growth, providing significant benefits in oncology settings.
Sterile, Ready-to-Use Formulation
Manufactured using strict aseptic techniques according to GMP guidelines, Cytarabine Injection is delivered as a sterile, pyrogen-free solution in glass vials. The ready-to-use liquid formulation ensures convenience and safety for both intravenous and subcutaneous administration by healthcare professionals.
Pharmaceutical Excellence and Compliance
This product meets international pharmacopeial standards, including USP, BP, and EP, ensuring consistent quality, efficacy, and safety. The formulations pH is carefully adjusted to a range of 7.08.5, supporting patient comfort and injection compatibility with standard IV fluids.
FAQs of Cytarabine Injection:
Q: How is Cytarabine Injection administered?
A: Cytarabine Injection is given either intravenously (IV) or subcutaneously (SC) as directed by a healthcare professional, depending on the prescribed treatment plan and clinical indication.Q: What cancers is Cytarabine Injection commonly used to treat?
A: Cytarabine Injection is primarily indicated for the treatment of certain leukemias (acute myeloid leukemia, acute lymphocytic leukemia, chronic myelogenous leukemia) and lymphomas.Q: When should Cytarabine Injection not be used?
A: This medicine should not be used in patients who are hypersensitive to Cytarabine or any component in the formulation. Any suspected allergy should be reported to the physician before use.Q: What are the storage requirements for Cytarabine Injection?
A: Cytarabine Injection should be stored in a cool place, following the manufacturers label instructions, to maintain the products effectiveness throughout its 24-month shelf life.Q: How does Cytarabine Injection benefit oncology patients?
A: Cytarabine works by inhibiting DNA synthesis in rapidly dividing cancer cells, which helps slow or stop the progression of leukemias and lymphomas, improving patient outcomes when used as part of a prescribed chemotherapy regimen.Q: Is Cytarabine Injection ready to use upon receipt?
A: Yes, the injection is supplied as a sterile, pyrogen-free liquid in a clear, colorless to pale yellow solution, ready for immediate use by trained medical personnel.Q: What standards and safety measures are followed in the manufacturing of Cytarabine Injection?
A: This injectable is produced using aseptic methods under Good Manufacturing Practice (GMP), and it meets stringent USP, BP, and EP standards for quality and safety.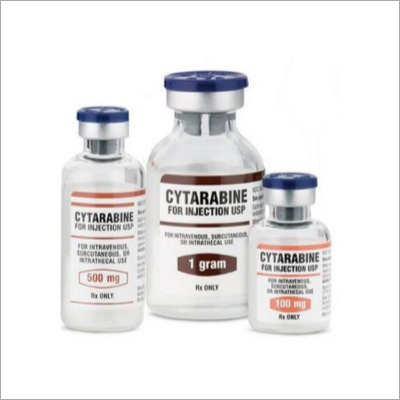

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Oncology Drugs Category
Granisetron HCl Tablets
Price 6-7 USD ($)
Minimum Order Quantity : 100 Boxes
Storage Instructions : Store in Cool
Formulations Form : Tablets
Application : Anti cancer
Shelf Life : 24 months
Lomustine Capsule
Price 8-9 USD ($)
Minimum Order Quantity : 100 Boxes
Storage Instructions : Store in Cool
Formulations Form : Capsules
Application : Other, Antineoplastic (chemotherapy) agent
Shelf Life : 24 months
Bortezomib Injection
Storage Instructions : Store at 2C to 8C (Refrigerated)
Application : Other, Anticancer Therapy
Shelf Life : 2 years
RITUXIMAB INJECTION
Price 50-100 USD ($)
Minimum Order Quantity : 1000 Boxes
Storage Instructions : Store in Cool
Formulations Form : Liquid
Application : Chemotherapy
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry