Ceftriaxone Tazobactam Injection
Ceftriaxone Tazobactam Injection Specification
- Formulations Type
- Injectables
- Formulations Form
- Powder
- Gender/Age Group
- Adult, Women
- Storage Instructions
- Store in Cool
Ceftriaxone Tazobactam Injection Trade Information
- FOB Port
- All Indian Port
- Payment Terms
- Paypal, Western Union, Telegraphic Transfer (T/T), Cash Advance (CA)
- Supply Ability
- 100000 Piece Per Month
- Delivery Time
- 7-10 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- As Per Buyer Requirements
- Main Export Market(s)
- Australia, North America, Africa, Western Europe, Central America, Eastern Europe, Middle East, South America, Asia
- Certifications
- ISO, GMP, WHO, COA
About Ceftriaxone Tazobactam Injection
Ceftriaxone Tazobactam Vial
We are a leading Manufacturer and Exporter of Ceftriaxone Tazobactam vial in different strength and packing. Our manufacturing facility is certified with WHO-GMP.
Cefoperazone-sulbactam combination in the treatment of urinary tract infections.
It is one of few cephalosporin antibiotics effective in treating Pseudomonas bacterial infections which are otherwise resistant to these antibiotics .
Composition:
- Ceftriaxone 1 Gm + Tazobactam 125 Mg
Feature:
- Product Type :Finished Product
- Dose/Strength: 1 gm + 125 mg
- type : vial
- Usage: Clinical,Hospital
Combination Therapy for Resistant Infections
By combining Ceftriaxone, a third-generation cephalosporin antibiotic, with Tazobactam, a beta-lactamase inhibitor, this injection offers broad-spectrum antibacterial action, making it highly effective against resistant strains.
Guidelines for Proper Storage and Handling
The injection is supplied in powder form and should be kept in a cool environment, as exposure to heat or moisture may reduce its therapeutic effectiveness or shelf life. Proper storage safeguards its potency until administration.
Trusted Exporter and Manufacturer in India
Produced in India by established manufacturers, this injection adheres to global standards to ensure quality, safety, and efficacy. It is supplied to healthcare facilities worldwide as a trusted solution for demanding clinical needs.
FAQs of Ceftriaxone Tazobactam Injection:
Q: How should Ceftriaxone Tazobactam Injection be administered?
A: The powder formulation is intended for reconstitution and administration via injection, typically by a healthcare professional. Dosage and frequency are determined based on the infection and patients condition.Q: What are the main benefits of combining Ceftriaxone with Tazobactam?
A: This combination provides broad-spectrum antibacterial activity, with Tazobactam protecting Ceftriaxone from beta-lactamase enzymes, thereby enhancing its effectiveness against resistant bacteria.Q: When is this injectable formulation recommended for use?
A: It is recommended for moderate to severe bacterial infections in adults, including women, when oral antibiotics are ineffective or not suitable, as directed by a physician.Q: Where should the Ceftriaxone Tazobactam Injection be stored?
A: The injection should be stored in a cool place, away from direct sunlight and moisture, to preserve its stability and potency until use.Q: What is the correct process for preparing the injectable from the powder?
A: A healthcare professional should reconstitute the powder with sterile water or specified diluent under aseptic conditions, following the manufacturers instructions to ensure proper mixing and dosing.Q: Who can benefit from this medication?
A: Adult patients and women with bacterial infections unresponsive to standard antibiotics can benefit from this injection, as prescribed by their doctor.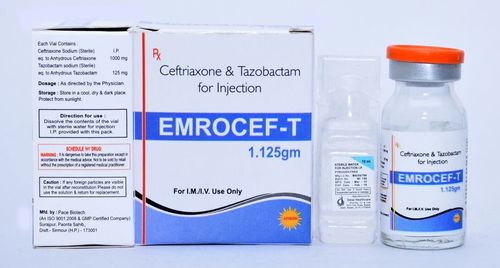

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Critical Care Products Category
Nelfinavir Tablets
Price 2-4 USD ($) / Box
Minimum Order Quantity : 500 Boxes
Storage Instructions : Dry Place
Gender/Age Group : Adult
Formulations Type : General Drugs
Formulations Form : Tablets
Paclitaxel Injection
Price 20 INR / Box
Minimum Order Quantity : 500 Boxes
Storage Instructions : Store in Cool
Gender/Age Group : Adult
Formulations Type : Injectables
Formulations Form : Liquid
Novoseven
Price 100-200 USD ($)
Minimum Order Quantity : 50 Boxes
Storage Instructions : Store in Cool
Gender/Age Group : Suitable For All Ages
Formulations Type : Injectables
Formulations Form : Liquid
Ganciclovir Injection
Price 5-10 USD ($)
Minimum Order Quantity : 500 Boxes
Storage Instructions : Store in Cool
Gender/Age Group : Other, Adult, Children
Formulations Type : Injectables
Formulations Form : Liquid
 |
REWINE PHARMACEUTICAL
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |


 Send Inquiry
Send Inquiry